TANOS®medical
systainer® in medical applications

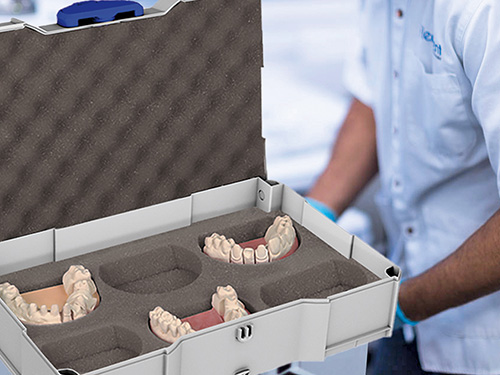
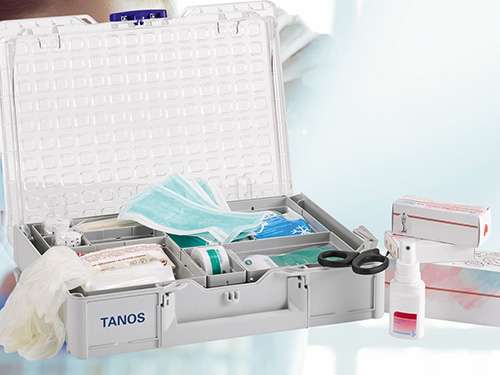
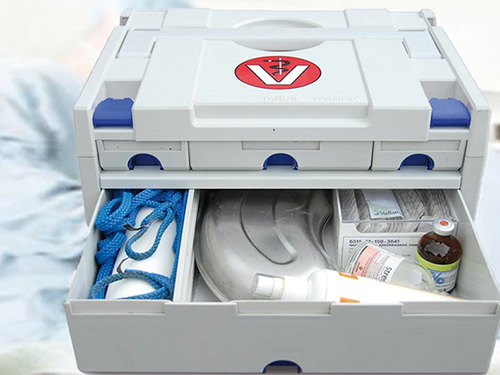
Security and reliability in transportation and handling are essential in the medical field.
A systainer® is the right choice, anywhere and anytime.
Each systainer® stands out by using high-quality ABS material and offers unbeatable properties:
- Shock-resistant
- Dimensionally stable in hot and cold conditions
- Protected against dust and moisture
- High chemical resistance to many diluted acids and bases, saturated hydrocarbons and gasoline
- Weather resistance
View other advantages of our systainer® here